Initial Indication: Head & Neck Cancer (HNC)
Growing market with significant unmet needs
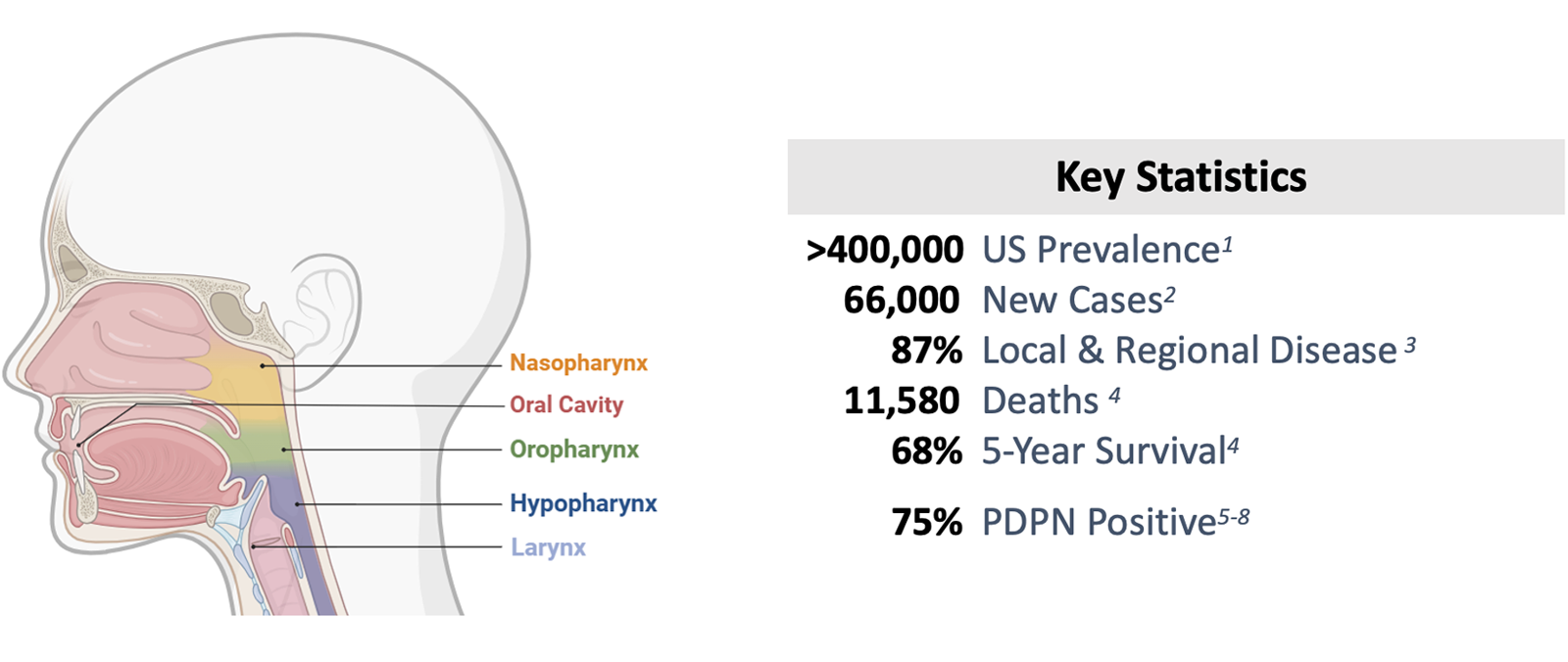
Sentrimed has prioritized Head and Neck Cancer (HNC) as an initial indication for MASL due to the market size and significant unmet needs. The global HNC therapy market has been estimated at $ 1.7 billion in 2022 and is projected to increase to $ 3 billion by 20309,10. In the U.S., more than 400,000 individuals have HNC, with 66,000 new cases and nearly 12,000 deaths annually. The five-year overall survival rate is 68% but varies greatly based on stage and location of the tumor. Recurrence occurs in 40% of patients11 primarily in the first 18 to 24 months with very poor outcomes. Surgery, radiation and/or chemotherapy are the standard of care treatments for oral cancer. Surgery and radiation can be disfiguring and have a significant impact on patients’ quality of life. There are very few approved therapeutic options for HNC, they have concerning warnings and precautions, and require intravenous dosing.
Sources:
- SEER*Explorer: SEER*Explorer: An interactive website for SEER cancer statistics [Internet]. Surveillance Research Program, National Cancer Institute; 2023 Apr 19. [updated: 2023 Nov 16; cited 2024 Jan 18];
- Head and Neck Cancer: Statistics | Cancer.Net; https://www.cancer.net/cancer-types/head-and-neck-cancer/statistics
- Sentrimed estimates
- Oral cavity and pharynx Statistics | American Cancer Society – Cancer Facts & Statistics; https://cancerstatisticscenter.cancer.org
- Oral Dis. 2016;22(4):303-312. doi:10.1111/odi.12442
- Histopathology. 2015;66(6):771-780. doi:10.1111/his.12496
- Molecular medicine reports vol. 6,2 (2012): 271-4. doi:10.3892/mmr.2012.911.
- Journal of clinical and diagnostic research : JCDR vol. 11,5 (2017): EC31-EC35. doi:10.7860/JCDR/2017/25939.9882
- Head and Neck Cancer Market: Global Industry Analysis and forecast 2029; (maximizemarketresearch.com)
- https://www.researchandmarkets.com/reports/5635839/global-head-and-neck-cancer-drugs-market-2022; Global Head & Neck Cancer Drugs Market (2022-2027) by Drug Class, Product, End User, Geography, Competitive Analysis and the Impact of Covid-19 with Ansoff Analysis
- Annals of oncology : official journal of the European Society for Medical Oncologyvol. 15,8 (2004): 1179-86. doi:10.1093/annonc/mdh308